- Select Language
-
-
 English
English
-
 Spanish
Spanish
-
 French
French
-
 German
German
-
 Italian
Italian
-
 Chinese (Simplified)
Chinese (Simplified)
-
 Japanese
Japanese
-
 Korean
Korean
-
 Arabic
Arabic
-
 Portuguese
Portuguese
-


 English
English
 Spanish
Spanish
 French
French
 German
German
 Italian
Italian
 Chinese (Simplified)
Chinese (Simplified)
 Japanese
Japanese
 Korean
Korean
 Arabic
Arabic
 Portuguese
Portuguese



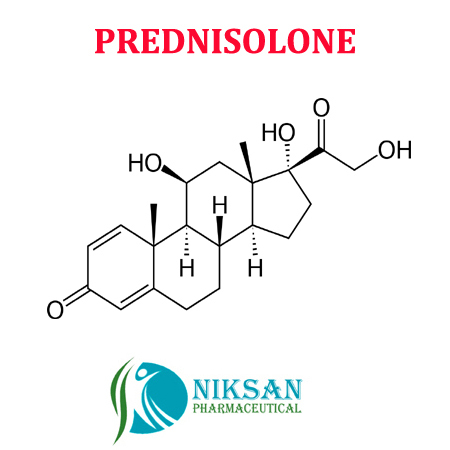



Niksan Pharmaceutical is one of the leading manufacturer, supplier, distributor and exporter of Prednisolone API and finished formulation as all dosage forms. Our product Prednisolone is widely used and appreciated by our group companies and also our customers and users all around the nations. We offer the Prednisolone in very affordable price.
Niksan Pharmaceutical is the suppliers,manufacturer, exporter and trader in the domestic level as well as the international market.
Niksan Pharmaceutical provides Prednisolone API in all over Indian states like Uttarakhand, Pondicherry, Kerala, Andhra Prades h, Punjab, HimachalPradesh, Chhattisgarh, Jammu & Kashmir, Haryana, Telangana, Goa, TamilNadu, Jharkhand, Karnataka, Rajasthan, Chandigarh, Gujarat, Maharashtra, UttarPradesh, Odisha, Delhi, West Bengal, Assam, Manipur, Bihar, Madhya Pradesh andmany other state.
Niksan Pharmaceutical also exportsPrednisolone API in world other countries like Zimbabwe, Trinidad & Tobago, Zambia, Ghana, Kenya,Singapore, Mauritius, Australia, Malaysia, Tanzania, Uganda, Nigeria, UnitedKingdom, Ireland, Puerto Rico, Iraq, Sri Lanka, France, Nepal, Sudan, Jordan,Thailand, Qatar, Oman, United Arab Emirates, Hong Kong, Taiwan, United States,Kuwait, Bangladesh, Saudi Arabia, Belgium, Pakistan, Morocco, Philippine,Algeria, Canada, New Zealand, South Africa, South Korea, Egypt, Norway,Indonesia, Iran, Switzerland, Vietnam, Greece, Denmark, Israel and many other countries.
Prednisolone is steroidmedicine which is used to treatment of allergies, inflammatory conditions,autoimmune disorders, and cancers.
SYNONYMS: Delta-Dehydrocortisol,Delta-Dehydrohydrocortisone, Delta-Hydrocortisone, Delta (1)-Dehydrocortisol,Delta (1)-Hydrocortisone, Hydroretrocortine, Metacortandralone, Prdl,Prednisolona, Prednisolone, Prednisolonum
IUPAC NAME: (1R,3aS,3bS,9aR,9bS,10S,11aS)-1,10-dihydroxy-1-(2-hydroxyacetyl)-9a,11a-dimethyl-1H,2H,3H,3aH,3bH,4H,5H,7H,9aH,9bH,10H,11H,11aH-cyclopenta[a]phenanthren-7-one
CAS NO: 50-24-8
FORMULA: C21H28O5
MOLECULAR MASS: 360.44 g/mol
APPLICATIONS OF Prednisolone: Prednisolone is used to treatment of immune system disorders, skin and eye conditions, arthritis, blood problems, breathing problem and cancer. It is used to treatment of some allergic reaction.
HOW TO USE: Take single dose in one day. Prednisolone is available as form of solutions and tablet so you can take by mouth with or without food.
HOW PrednisoloneWORKS: Prednisolonework by lowering the activity of the immune system.
CONTRAINDICATIONS: If you have any untreated tuberculosis, inactive tuberculosis, herpessimplex infection of the eye, a herpes simplex infection and an infection due to a fungus kindly avoid Prednisolone. If you have low amount of potassium or magnesium in your blood or body kindly avoid Prednisolone.
PHARMACOKINETICS OF Prednisolone: Absorption is approximately 70%after oral administration. Half life of Prednisolone is 2.1 to 3.5 hours. Peak plasma concentration of Prednisolone is approximately 113-1343mg/ml. 98%Prednisolone eliminate via urine.
SIDE EFFECTS OF Prednisolone: Common side effect of Prednisolone is weight gain, Indigestion,sleep problems, Restlessness and sweating a lot.
PRECAUTIONS: Before using this medication, tell your doctor or pharmacist your medical history. This drug may make you dizzy so avoid alcohol and do not drive and do not handle machinery. In the rare case you may infected by Prednisolone.This medication may effect on children's height.
CDSCO APPROVAL: Levofloxacin(15mg) + Prednisolone Acetate 10mg per ml eye drops are approved by CDSCO in India in 11.07.2007
Clotrimazole(10mg) + Methylprednisolone aceponate 1mg/gm of cream is approved by CDSCO in India in 12.07.2007
Docetaxal injection is approved by CDSCO in India in 09.03.2005
MoxifloxacinHCl (5mg) + Prednisolone Acetate (10mg) per ml. (eye drops) are approved by CDSCO in India in 16.10.2007
Prednisolone stearoyl glycolate tablet is approved by CDSCO in India in November-1970
Mupirocin(2%) + Methyl prednisolone Aceponate (0.1%) Cream is approved by CDSCO in India in31.10.2007
Methylprednisolone aceponate-1mg + salicylic acid-50mg tablet is approved by CDSCO in India in 19.11.2007
Methyl prednisolone aceponate-1mg + Mupirocin-20mg cream is approved by CDSCO in India in 21.11.2007
Prednisolone Sodium Phosphate Orally Disintegrating Tablet is approved by CDSCO in India in26.11.2009
Prednisolone tablet is approved by CDSCO in India in December-1971
Methyl prednisolone tablet is approved by CDSCO in India in March-1978
Methyl prednisolone acetate tablet is approved by CDSCO in India in November-1962
Methyl prednisolone Tablet 8mg is approved by CDSCO in India in 01.09.2009
Prednisolone+ Ofloxacin tablet is approved by CDSCO in India in 2003
Methylprednisolone Acrponate topical solution 0.1%is approved by CDSCO in India in 09.04.2007
Gatifloxacin( 0.3%) + Prednisolone Acetate (1%) eye drops are approved by CDSCO in India in 02.06.2006
Prednisolone stearoylglycolate tablet is approved by CDSCO in India in February-1971
MethylPrednisolone Aceponate Cream (0.1%) is approved by CDSCO in India in 15.06.2006
Lincomycin +Neomycin + Methyl prednisolone is approved by CDSCO in India inMarch-1989
FORMULATIONS AVAILABLE IN MARKET:
Prednisolone 10 MG tablets
Prednisolone 5 MG tablets
Prednisolone 1 %W/V solutions
Prednisolone 30 MG tablets
Prednisolone 20 MG tablets
Prednisolone 40 MG tablets
Prednisolone 1 MG tablets
Prednisolone 2 MG tablets
Ofloxacin 3MG+Prednisolone 10 MG /ML solutions
Prednisolone 5 MG /5ML solutions
Prednisolone 15 MG /5MLsolutions
Prednisolone 2.5 MG tablet
Moxifloxacin 0.5 %W/V+Prednisolone 1 %W/V solutions
Gatifloxacin 0.3 %W/V+Prednisolone 1 %W/V solutions
Chloramphenicol 0.5 %W/V+Prednisolone 0.1 %W/V solutions
Note: Product protected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk.
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult



 |
NIKSAN PHARMACEUTICAL
All Rights Reserved. (Terms of Use) Developed and Managed by Infocom Network Private Limited. |
天下网标王深圳小产权房网站优化建材网站优化平台南宁网站运营优化公司如何提高网站优化滁州网站长尾关键词优化黄州区网站关键词排名优化价格高新网站优化哪家好新塘网站权重优化奎屯网站优化推广大东区数据网站建设优化价格谢岗镇网站优化中山手机网站优化方案普洱网站优化运营郑州靠谱的服务行业网站优化优化seo网站网站页面代码优化的方法有哪些呢无锡做网站优化哪家公司靠谱安宁网站优化排名临沂哪里有网站优化价格优化网站免费咨询亳州企业网站排名优化哪家专业网站优化方案如何写手表网站内部优化方案重庆网站seo优化报价雅安企业网站优化北京网站优化排名平台收费标准龙山优化网站最新报价荆门很好的网站优化用户体验五桂山网站优化南充优化网站咨询香港通过《维护国家安全条例》两大学生合买彩票中奖一人不认账让美丽中国“从细节出发”19岁小伙救下5人后溺亡 多方发声卫健委通报少年有偿捐血浆16次猝死汪小菲曝离婚始末何赛飞追着代拍打雅江山火三名扑火人员牺牲系谣言男子被猫抓伤后确诊“猫抓病”周杰伦一审败诉网易中国拥有亿元资产的家庭达13.3万户315晚会后胖东来又人满为患了高校汽车撞人致3死16伤 司机系学生张家界的山上“长”满了韩国人?张立群任西安交通大学校长手机成瘾是影响睡眠质量重要因素网友洛杉矶偶遇贾玲“重生之我在北大当嫡校长”单亲妈妈陷入热恋 14岁儿子报警倪萍分享减重40斤方法杨倩无缘巴黎奥运考生莫言也上北大硕士复试名单了许家印被限制高消费奥巴马现身唐宁街 黑色着装引猜测专访95后高颜值猪保姆男孩8年未见母亲被告知被遗忘七年后宇文玥被薅头发捞上岸郑州一火锅店爆改成麻辣烫店西双版纳热带植物园回应蜉蝣大爆发沉迷短剧的人就像掉进了杀猪盘当地回应沈阳致3死车祸车主疑毒驾开除党籍5年后 原水城县长再被查凯特王妃现身!外出购物视频曝光初中生遭15人围殴自卫刺伤3人判无罪事业单位女子向同事水杯投不明物质男子被流浪猫绊倒 投喂者赔24万外国人感慨凌晨的中国很安全路边卖淀粉肠阿姨主动出示声明书胖东来员工每周单休无小长假王树国卸任西安交大校长 师生送别小米汽车超级工厂正式揭幕黑马情侣提车了妈妈回应孩子在校撞护栏坠楼校方回应护栏损坏小学生课间坠楼房客欠租失踪 房东直发愁专家建议不必谈骨泥色变老人退休金被冒领16年 金额超20万西藏招商引资投资者子女可当地高考特朗普无法缴纳4.54亿美元罚金浙江一高校内汽车冲撞行人 多人受伤